Lecture 4: Krebs Cycle and the Electron Transport Chain
June 14, 2012 § Leave a comment
Contents
- Mitochondrial reactions
- Krebs citric acid cycle
- Krebs cycle summary
- Introduction to electron transfer
- Electron transport chain
- ETC overview
Mitochondrial reactions
After glycolysis, the pyruvate and NADH products are moved from the cytoplasm to the mitochondria, in order to proceed with the Krebs cycle and oxidative phosphorylation. The mitochondrion is the energy producing organelle. It originated from independent bacteria living within bigger cells in symbiosis. This is the reason why the mitochondria in a cell have their own DNA, separate from the DNA in the nucleus, as well as the reason why mitochondria have a double membrane. The outer mitochondrial membrane (OMM) is porous. As long as a molecule is less than 1000 amu, it can freely pass this barrier. The inner mitochondrial membrane (IMM), is the true barrier – a molecule requires a membrane bound transporter to pass through this membrane. The space in the organelle past the IMM is known as the matrix, and the folds formed by the IMM is known as the cristae.
The Krebs cycle occurs in the matrix of the mitochondria. A pyruvate transporter in the IMM enables the three-carbon product of glycolysis to pass through. The electron transport chain reactions that occur afterwards rely on the IMM to hold the complexes in place, and maintain the concentration gradient required for ATP synthesis.
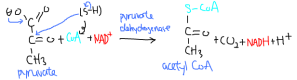
Before entering the Krebs cycle, the pyruvate has to be further modified into acetyl CoA. This requires the enzyme pyruvate dehydrogenase. This is an enzyme complex with three catalytic subunits. It catalyzes the decarboxylation of pyruvate, replacing the carboxyl group with the CoA group (CoA-S.) PDH is a oxidoreductase, as it also requires NAD+. Overall, pyruvate is oxidized into acetyl CoA, and NAD+ is reduced into NADH. The NADH product will be used in the ETC to generate ATP. The carboxyl group released turns into a CO2 molecule, and this is a waste product. The reaction is very favourable at -33 kJ/mol. PDH is controlled by feedback inhibition. ATP is an allosteric inibitor, and its products, acetyl CoA (on subunit 2) and NADH (on subunit 3) are competitive inhibitors for its active site. It can also be deactivated/activated by covalent modification. PDH kinase uses ATP to phosphorylate and deactive PDH. PDH phosphotase does the opposite. PDH kinase can also be activated by the products acetyl CoA and NADH, and inhibited by pyruvate and ADP. Ca+2 activates PDH phosphatase during muscle activity.
Pyruvate + CoA + NAD+ -> Acetyl CoA + CO2 + NADH + H+
Catalyzed by pyruvate dehydrogenase, activated by PDH phosphatase (itself activated by Ca+2), inhibited by acetyl CoA (E2), NADH (E3), ATP, PDH kinase (itself actiavted by NADH, acetyl CoA, inhibited by pyruvate, ADP)
Krebs citric acid cycle
Now that pyruvate has been converted into acetyl CoA, it can enter the Krebs cycle. It joins with the last molecule of the previous cycle, oxaloacetate, to form a six carbon compound. Through the cycle, it loses carbons in the form of CO2 until it is oxidized into the four carbon oxaloacetate once again. NADH, FADH2, and GTP are also produced as products released from the cycle. These all can be converted into ATP or provide the energy for ATP synthesis. The reactions are summarized below:
- Citrate synthase reaction
- Aconitase synthase reaction
- Isocitrate dehydrogenase reaction
- α-Ketoglutarate dehydrogenase reaction
- Succinyl CoA synthetase reaction
- Succinate dehydrogenase reaction
- Fumarase reaction
- Malate dehydrogenase reaction
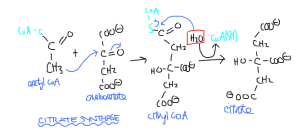
The first reaction is the citrate synthase (a lyase, even though it requires water) reaction. This enzyme combines oxaloacetate and acetyl CoA, first into the citryl CoA intermediate, which then becomes the six carbon citrate. The methyl group of acetyl CoA is the first to reaction, attacking the ketocarbon of oxaloacetate. Once joined, the energetic thioester linkage between C and S will be hydrolyzed by H2O, releasing the entire CoA group, leaving citrate. Citrate, although it has six carbons, is achiral, and contains three carboxyl groups. The energy for the reaction is -31 kJ/mol.
Oxaloacetate + acetyl CoA -> Citryl CoA -> Citrate
Catalyzed by citrate synthase
Aconitase synthase catalyzes the next reaction. Citrate loses a hydrogen and hydroxyl group (lost in the form of H2O) to form a double bond between two carbons. This is the cis-aconitate. The H2O enters again to reattaches to a different carbon, becoming isocitrate. This isomer of citrate is chiral. The reaction is not exactly favourable, so it requires concentration manipulation at equilibrium position.
Citrate ⇌ cis-Aconitate + H2O ⇌ Isocitrate
Catalyzed by aconitase synthase
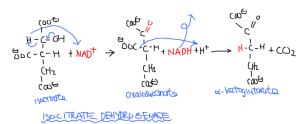
The isocitrate is then decarboyxlated by isocitrate dehydrogenase (ICDH), another oxidoreductase. It is the first site of NADH production in the Krebs cycle, as isocitrate becomes oxalosuccinate after losing a hydrogen from the hydroxyl group (thus forming a C=O ketone group.) The keto-carboxyl groups are highly energetic when adjacent, so a carboxyl group is lost as CO2 and α-ketoglutarate, a five carbon compound is the product.
Isocitrate + NAD+ -> Oxalosuccinate + NADH + H+ -> α-Ketoglutarate + CO2
Catalyzed by isocitrate dehydrogenase
Another oxidative decarboxylation follows, using the enzyme α-ketoglutarate dehydrogenase (α-KGDH). The carbon with both ketone and carboxyl groups loses the carboxyl group (as CO2,) and it is replaced by CoA. It is the second site of NADH production. The S-CoA (thioester) bond plus ketone C=O bond on the same carbon resembles a carboxyl group, as S is quite similar to O. Note that by this reaction, the product is a four carbon compound – the same as the oxaloacetate of the first reaction. There will be no more removals of carbons from the substrate after this reaction. Dehydrogenase reactions will remove hydrogens or hydroxyl groups instead of carboxyl groups. The product is called succinyl CoA, and its hydrolysis generates -33 kJ/mol – even greater than ATP -> ADP! This is the basis of the next reaction.
α-Ketoglutarate + NAD+ + CoA -> Succinyl CoA + CO2 + NADH
Catalyzed by α-ketoglutarate dehydrogenase
To break the energetic thioester bond and harvest the energy, succinyl CoA synthetase (a ligase) is used. The CoA group is removed, reforming a carboxyl group, making succinate. The breaking of this bond provides the energy to phosphorylate GDP into GTP. This reaction is potentially reversible, at an energy change of -3.3 kJ/mol. GTP can react with ADP to pass its phosphate group with the enzyme nucleoside diphosphokinase, making ATP and GDP. The energy on an even plane at 0 kJ.
Succinyl CoA + Pi + GDP -> (potentially reversible) Succinate + CoA(SH) + GTP
Catalyzed by succinyl CoA synthetase
The succinate is then taken by succinate dehydrogenase (SDH) to use in another redox reaction. It is important to note that SDH is the only enzyme of the Krebs cycle that is attached as a membrane bound protein onto the IMM (the rest float in the matrix.) This is to later facilitate the passing of FADH2 into the ETC via CoQ. SDH removes two hydrogens from succinate to transfer the energy into FADH2, forming a double bond between the two carbons – this is fumarate.
Succinate + FAD -> Fumarate + FADH2
Catalyzed by succinate dehydrogenase
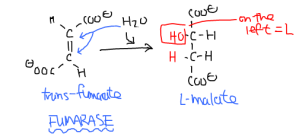
The penultimate reaction is the fumarase reaction. Adding water to fumarate, an achiral molecule, breaks the C=C double bond to add a hydroxyl group. This forms chiral malate (usually L-malate.)
Fumarate + H2O -> Malate
Catalyzed by fumarate
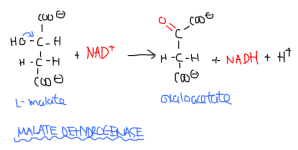
Malate dehydrogenase takes the malate through the last reaction of the Krebs cycle. Since it is the last reaction, it must produce a substrate for the first reaction to continue the cycle. Thus, MDH converts malate into oxaloacetate by removing a hydrogen from a hydroxyl, thus forming a C=O bond ketone. It is the third and last site of NADH production. The reaction is highly unfavourable at +29.7 kJ/mol and is managed by adjusting concentration. Oxaloacetate then returns to the first reaction (citrate synthase.)
Malate + NAD+ -> Oxaloacetate + NADH + H+
Catalyzed by malate dehydrogenase
The Krebs cycle occurs for each pyruvate. Thus, for each glucose, there are two turns of the cycle!
Krebs cycle summary
To summarize, there are two exit points of CO2, two entry points of H2O, three exits for NADH, one exit for FADH2, and one exit for GTP. The equation is below (note GTP is recorded as immediately converted to ATP):
Acetyl CoA + 2H2O + 3 NAD+ + FAD + ADP + Pi -> 2 CO2 + 3 NADH + FADH2 + CoA(SH) + ATP
The two exit points for CO2 are at two dehydrogenase reactions – isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. Water is needed for the first reaction, citrate synthase, and the second last fumarase reactionl. NADH is produced during the isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and malate dehydrogenase reactions. FADH2 is made in the succinate dehydrogenase reaction. Lastly, the GTP exit point is at the succinyl CoA synthetase reaction.
There are several regulatory points for the Krebs cycle as well:
- Citrate synthase: inhibited by NADH (products of Krebs cycle) and ATP (energy index)
- Isocitrate dehydrogenase: inhibited by NADH (its own product) and ATP (energy index), activated by ADP (energy index)
- α-Ketoglutarate dehydrogenase: inhibited by NADH (its own product) and succinyl CoA (own product), activated by AMP (energy index)
Introduction to electron transfer
After the Krebs cycle, the NADH and FADH2 proceed to transfer their electrons down an energy gradient to reduce other molecules of decreasing energy. The final reduction is the reduction of O2 into H2). The energy gathered powers the proton gradient that drives ATP synthase. The ETC energy pumps H+ from the matrix into the intermembranal space, creating the proton gradient that drives ATP synthesis. The ETC can be visualized as a sloping “bucket brigade”, where every further bucket is has a lower reduction potential and energy level, but increasing electron affinity. The bucket that currently holds the water would be the reduced complex carrying the electrons – all the other buckets are empty at that moment, and thus oxidized. The entire transfer of electrons from NADH into H2O has an energy change of -220 kJ.
Each electron transfer that occurs has an overall change in standard reduction potential, Eo. Change can be calculated as:
ΔEo = Eoacceptor – Eodonor
The more negative the Eo value, the better the reducing agent. Thus, throughout the ETC, each next complex/compound has an increasingly positive Eo. ΔEo is always positive for the ETC reactions.
Electron transport chain
The ETC is comprised of four complexes and two other compounds, ubiquinone (Q, or CoQ) and cytochrome c. NADH and FADH2 enter the chain to donate their electrons. It can be seen that Q and cytochrome c are intermediate carriers of electrons between complexes, and each interfaces with two complexes. CoQ accepts the electrons from complex I and II to pass to III, and cytochrome c accepts from III to pass to IV. The four complexes are:
- NADH-Q reductase
- Succinate-Q reductase
- Q-cytochrome c reductase
- Cytochrome c oxidase
Complex I facilitates the transfer sequence NADH -> FMN -> CoQ. It is called the NADH-Q reductase complex. NADH and an extra proton will transfer two electrons to flavin mononucleotide (FMN) to create FMNH2. In reduced form, two N=C bonds are broken to attach an H+ and electron to each. It is very similar to FADH2. FMNH2 then transfers the protons and electrons to ubiquinone (CoQ). The reduced form of CoQ is CoQH2, and two C=O bonds are broken to accomodate the protons and electrons. Eo for NAD+/NADH is -0.32V, and +0.03V for CoQ/CoQH2, totalling a ΔEo of +0.35V, a very big drop.
NADH + H+ + FMN -> NAD+ + FMNH2
FMNH2 + CoQ -> FMN + CoQH2
Unlike the free NADH from glycolysis and the Krebs cycle, FADH2, even after reduction, remains attached to succinate dehydrogenase. This is why SDH is bound to the IMM. Complex II, the succinate-Q reductase drives the FADH2 to reduce CoQ. Thus, FAD remains attached to succinate dehydrogenase, this SDH-FAD complex can proceed to catalyze more reactions with succinate in the Krebs cycle. Overall, the net equation involving succinate, FAD, and CoQ would result in succinate + CoQ -> fumarate + CoQH2. The Eo of succinate is +0.03V and +0.045V for CoQ. Thus, the drop in reduction potential is minor, and the energy generated in this step cannot produce ATP.
FADH2 + CoQ -> FAD + CoQH2
Complex I transfers electrons from NADH to CoQ. Complex II transfers electrons from FADH2 to CoQ. Now, in complex III, Q-cytochrome c oxidoreductase, it receives all the electrons captured by CoQ to pass onto cytochrome c. Cytochromes are ETC agent proteins, with an active heme A prosthetic group. The group contains iron, in Fe3+ (ferric) state when oxidized. When reduced, it becomes Fe2+ (ferrous). Each cytochrome contains one iron/heme so it can only carry one electron. Thus, each CoQH2 requires two cytochrome c to accept its electrons. Previously, H+/protons were transferred along with the electrons. However, due to the reduction managed by Fe, protons have no place at complex III and are released. The ΔEo for this reaction is +0.19V, another big drop (cytochrome c has a +0.235V potential.) In fact, there is an intermediates of cytochrome b and c1 within the complex as well, before the electrons are passed to Fe:
CoQH2 + 2 cytochrome b Fe3+ -> CoQ + 2 cytochrome b Fe2+
2 cytochrome b Fe2+ + 2 cytochrome c1 Fe 3+ -> 2 cytochrome b Fe3+ + 2 cytochrome c1 Fe 2+
2 cytochrome c1 Fe2+ + 2 cytochrome c Fe 3+ -> 2 cytochrome c1 Fe3+ + 2 cytochrome c Fe 2+
Complex IV is cytochrome c oxidase. Electrons pass from cytochrome c to cytochrome a, a3, then finally O2. Cytochrome c donates electrons to cytochrome a via copper components. It then passes to a, a3, then O2. Note that each transfer of two electrons technically requires half a O2 (one H2O only has one oxygen molecule.) To reduce one whole O2, four cytochrome c molecules are required. The ΔEo here is the strongest of all ETC reactions, at +0.585V (O2 has a potential of +0.82V.) The overall net reaction is stated below:
4 cytochrome c Fe2+ + 4 H+ + O2 -> 2 H2O + 4 cytochrome c Fe 3+
ETC overview
Recall that proton pumping is powered by the energy generated from electron transfer.
- 4 protons are pumped from complex I
- 0 protons are pumped from complex II
- 4 protons are pumped from complex III
- 2 protons are pumped from complex IV
Each NADH produces 2.5 ATP. The P/O ratio is thus 2.5 – there are 2.5 Pi per 1 O (that is, 1/2 O2.) Since the hydrolysis of ATP produces -30 kJ/mol, the opposite reaction (formation of ATP) requites 75 kJ of energy to make 2.5 ATP. Recall that the ETC generates -220 kJ per NADH processed. This means that only 34% of the energy was conserved to be made into ATP. The rest is released as heat. In addition, since each reverse reaction of hydrolysis creates one H2O, and in the complex IV reaction, one H2O is made per half O2, 3.5 molecules of H2O are formed in the generation of 2.5 ATP.
Ratio of NADH : O2 : ATP : H2O is 1 : 0.5 : 2.5 : 3.5
In comparison, FADH2 does not generate as much energy. The P/O ratio is only 1.5.
Ratio of FADH2 : O2 : ATP : H2O is 1 : 0.5 : 1.5 : 2.5
There are inhibitors for the ETC as well. Amytal, a sleeping barbituate blocks electron flow from FMNH2 to CoQ. Rotenone, an insecticide, does the same. Antimycin, from mold, blocks flow from cytochrome b to cytochrome c. Lastly, compounds such as hydrogen sulfide (H2S), cyanide (CN–), carbon monoxide (CO), and azide (N3) can block the reduction of O2 in the last step from cytochrome a3.






Leave a comment